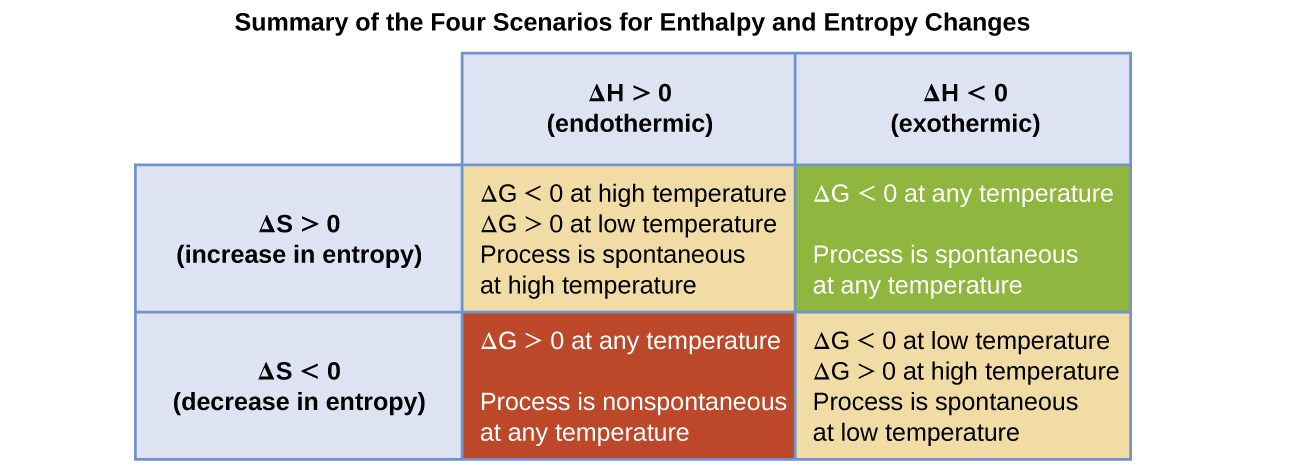
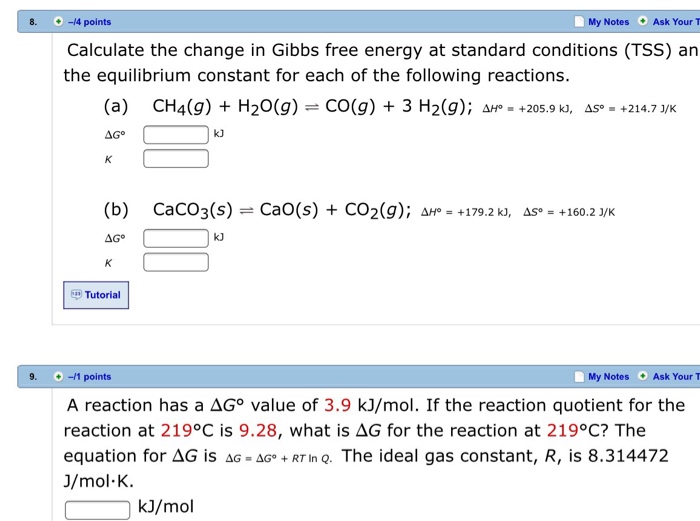
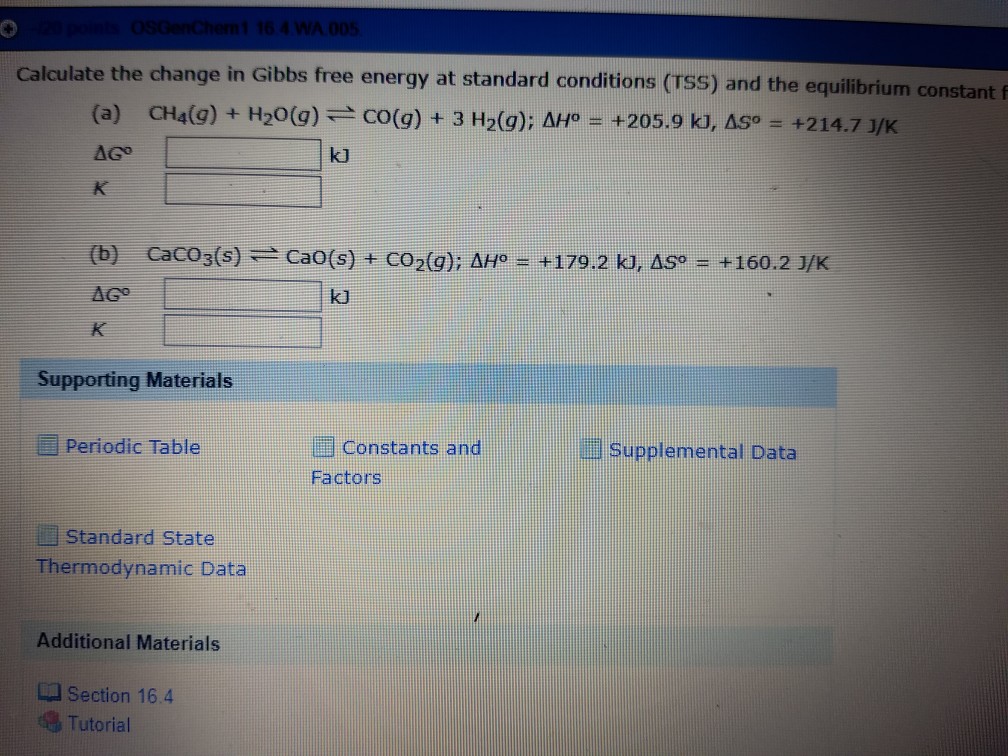
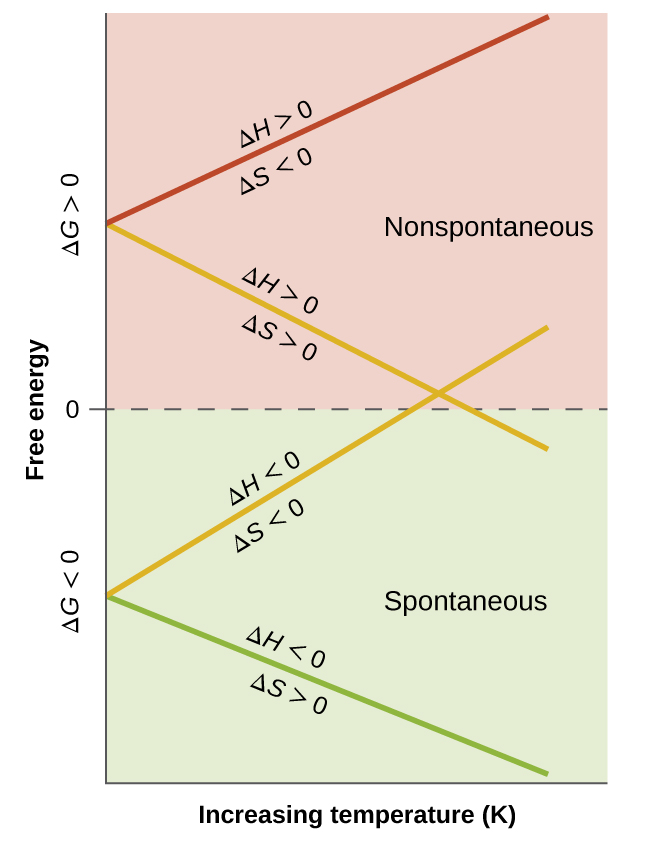
You must convert your standard free energy value into. Gibbs free energy denoted G combines enthalpy and entropy into a single value.

17 1 Equilibrium And Gibbs Free Energy Hl Youtube
G H TS.

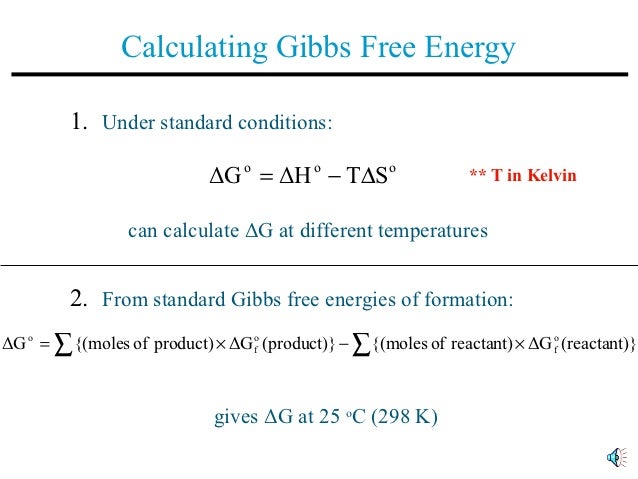
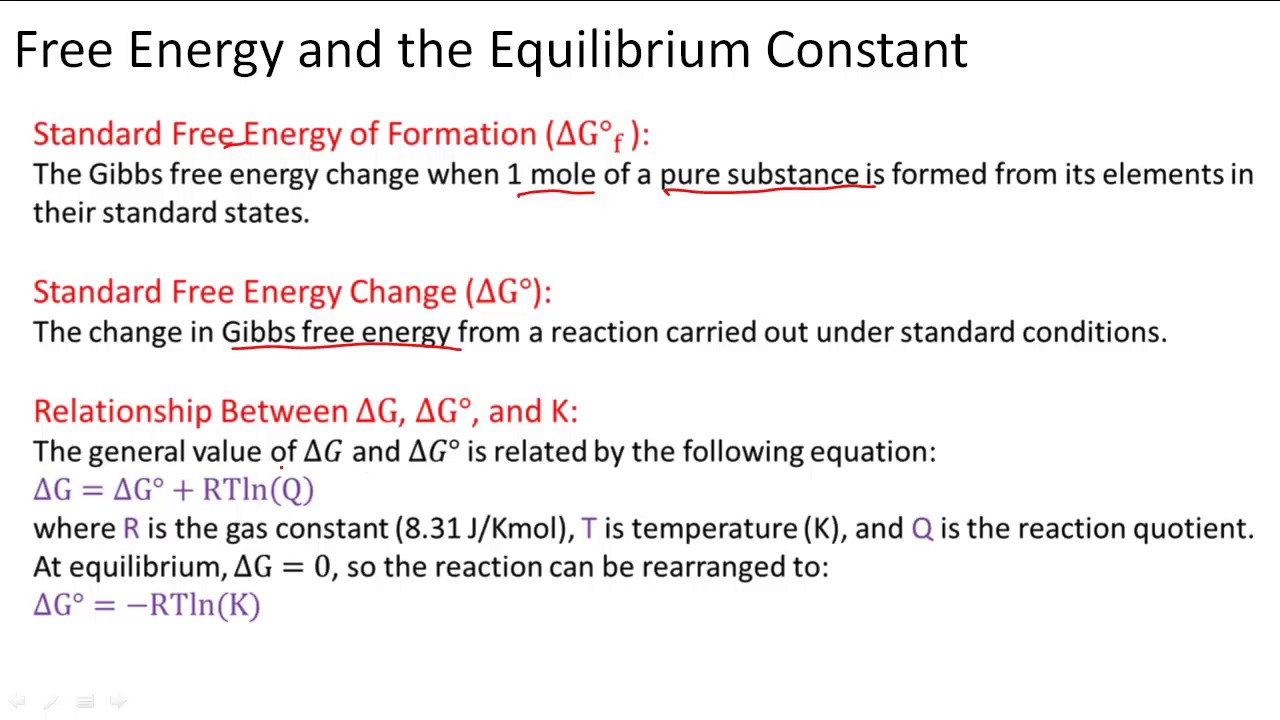
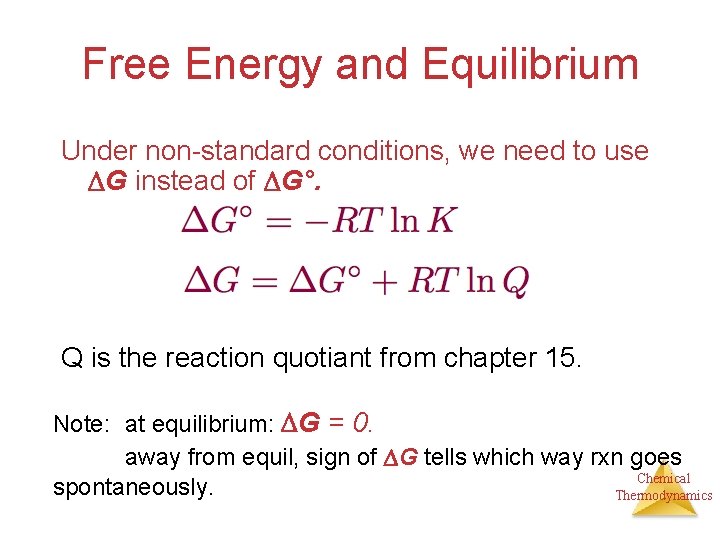
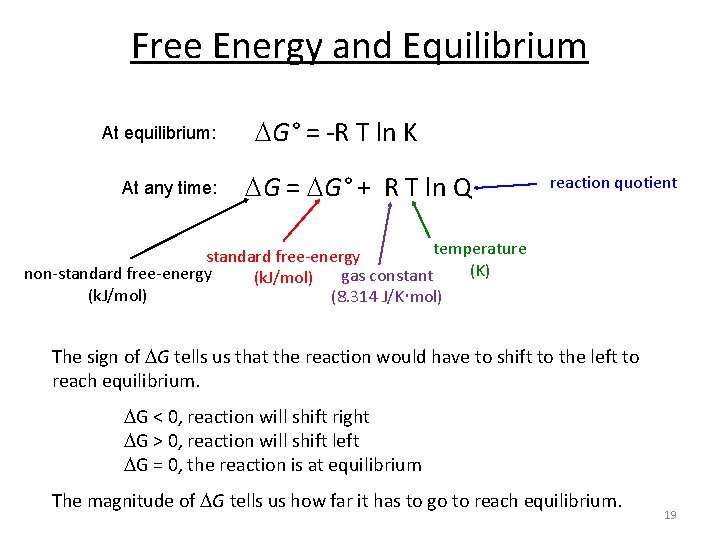
Gibbs free energy equation standard conditions. Conversely if ΔG 0 then K 1 and reactants are favored over products at equilibrium. The change in Gibbs free energy under nonstandard conditions Δ G can be determined from the standard change in Gibbs free energy Δ G ⁰. T is the temperature on the Kelvin scale.
ΔG ΔH - TΔS ΔG -8904 - 298-02442 -8176 kJ mol-1 It is easy as long as you remember to convert the entropy change value into kJ. A B C D. When a system changes from an initial state to a final state the Gibbs free energy ΔG equals the work exchanged by the system with its surroundings minus the work of the pressure force.
The change in the Gibbs free energy of the system that occurs during a reaction is therefore equal to the change in the enthalpy of the system minus the change in the product of the temperature times the entropy of the system. Ln Q is the natural logarithm of the reaction quotient. The first statement is consistent with the definition of standard states.
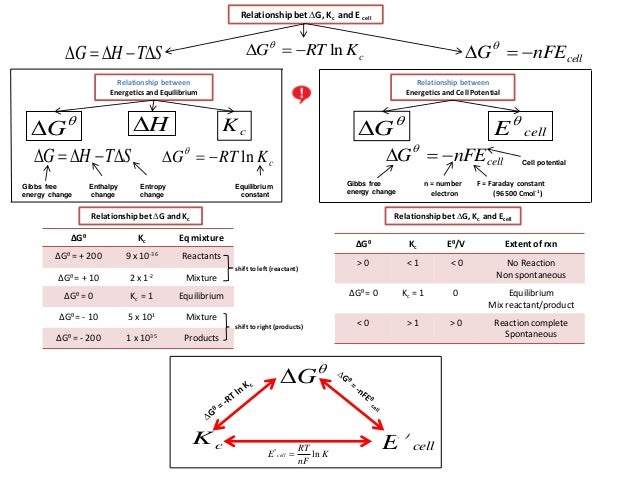
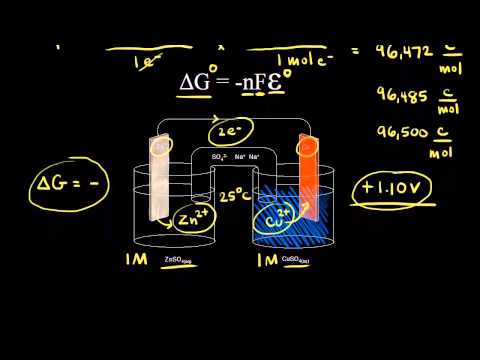
The free energy change D G is equal to -T D S univ and it applies just to a system itself without regard for the surroundings. Where R is the ideal gas constant 8314 Jmol K Q is the reaction quotient and T is the temperature in Kelvin. 1 E o E r e d u c t i o n o E o x i d a t i o n o Δ G is also related to E under general conditions standard or not via 2 Δ G n F E.
G H - TS If the reaction is run at constant temperature this equation can be written as follows. R 8314 J mol-1K-1or 0008314 kJmol-1K-1. It is defined by the Gibbs equation.
129 rows The standard Gibbs free energy of formation Gf of a compound is the change of Gibbs. D G D H - T D S. Looking at the below equation we can assume if the reaction is reversible and the Gibbs free energy is zero.
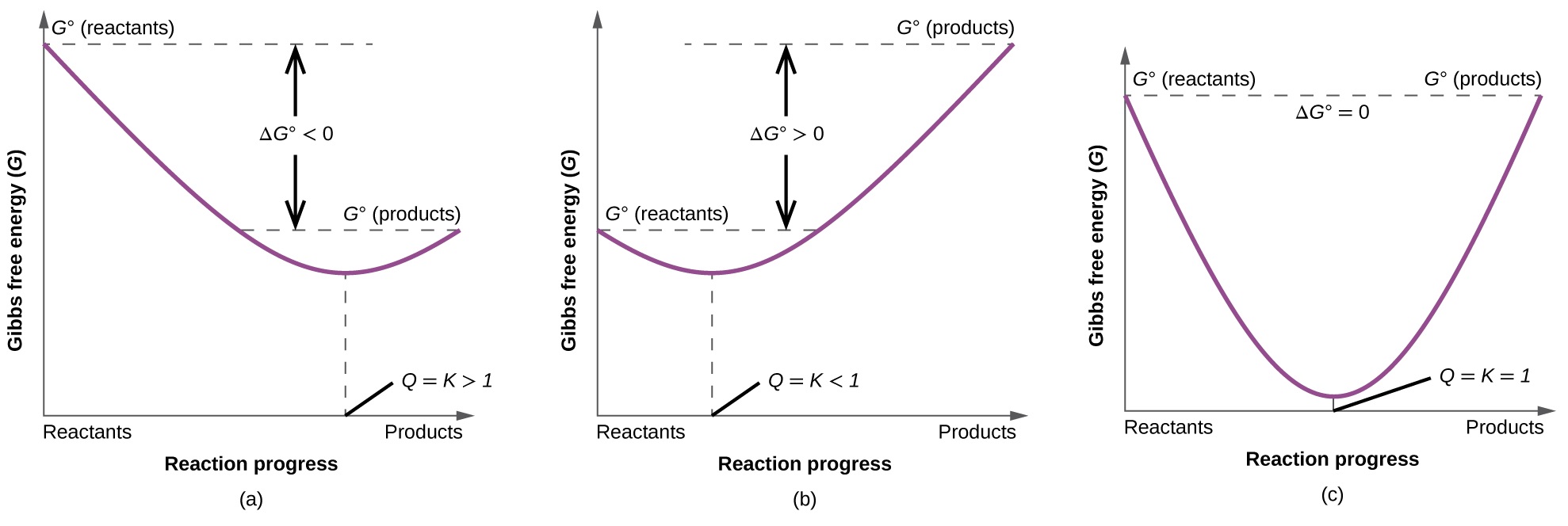
Gibbs Free Energy Equation Gibbs free energy is equal to the enthalpy of the system minus the product of the temperature and entropy. G is the change of Gibbs free energy for a system and G is the Gibbs energy change for a system under standard conditions 1 atm 298K. At equilibrium Q K Δ G is the free energy change for conversion of reactants to products in their standard states.
So if you had to calculate the Gibbs free energy change at say 298 K you can just slot the numbers in. If the products and reactants are in their standard states and ΔG 0 then K 1 and products are favored over reactants at equilibrium. Using Standard Change in Gibbs Free Energy Δ G ⁰.
ΔG can predict the direction of the chemical reaction under two conditions. The change in free energy ΔG is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system. We can say that the system is in equilibrium.
Delta H ΔH is the enthalpy change in kilojoules per mole KJmole the temperature is measured in Kelvin and the entropy change is measured in joules per kelvin per mole. Or the total change in any of the property is zero. Gibbs free energy equation.
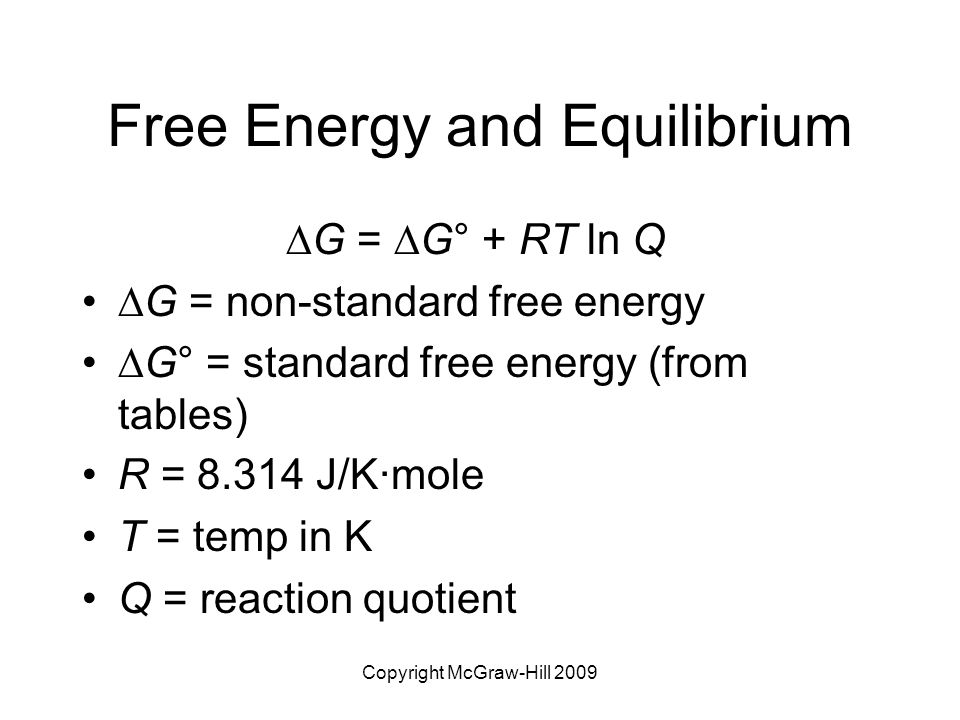
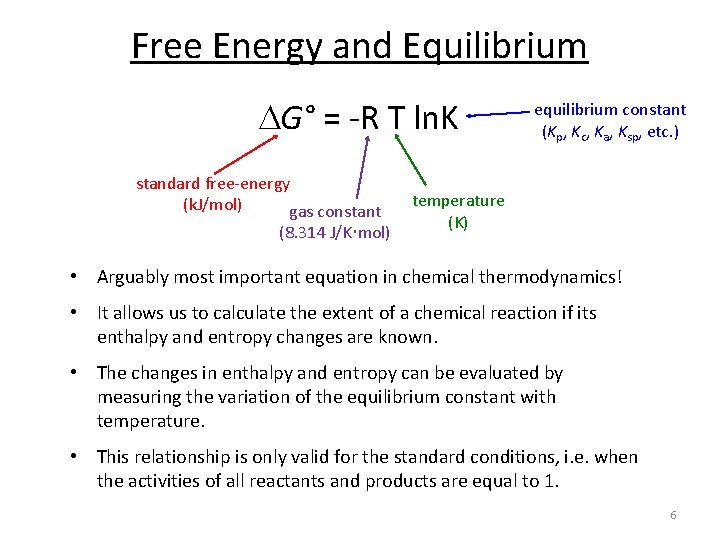
G G n R T ln. The Nernst Equation is derived from the Gibbs free energy under standard conditions. RG 0 The Gibbs energy for a reaction which is in the standard state rGᶿ is related to the equilibrium constant as follows.
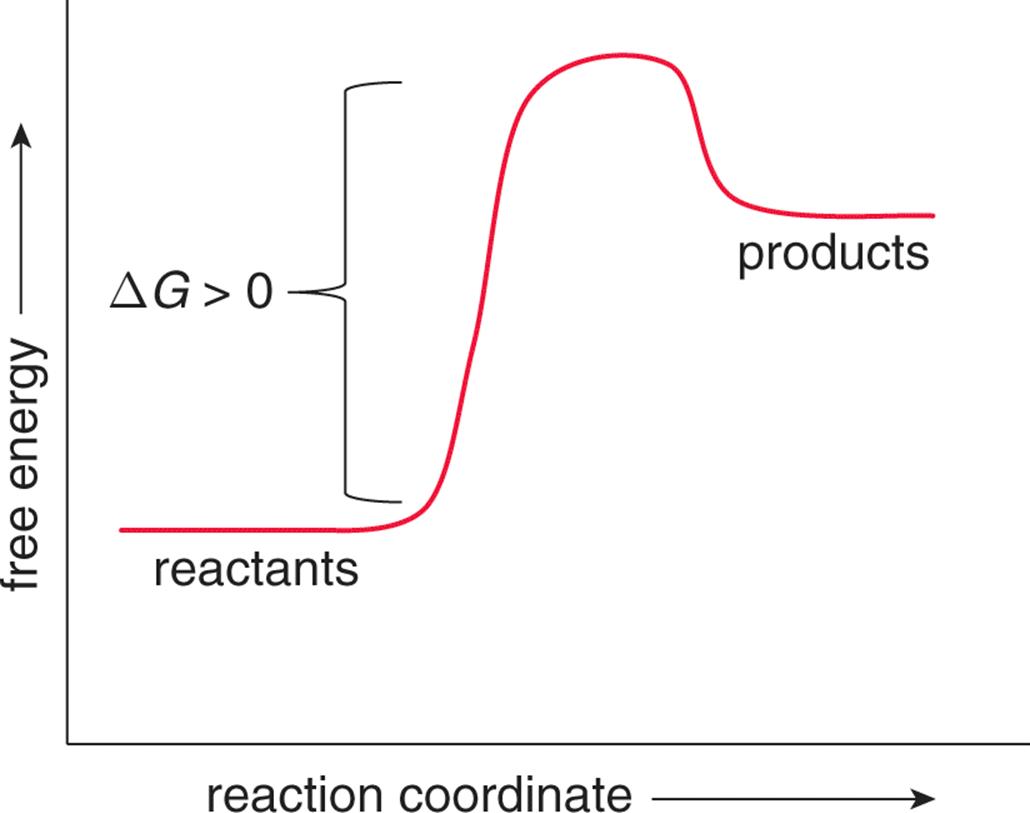
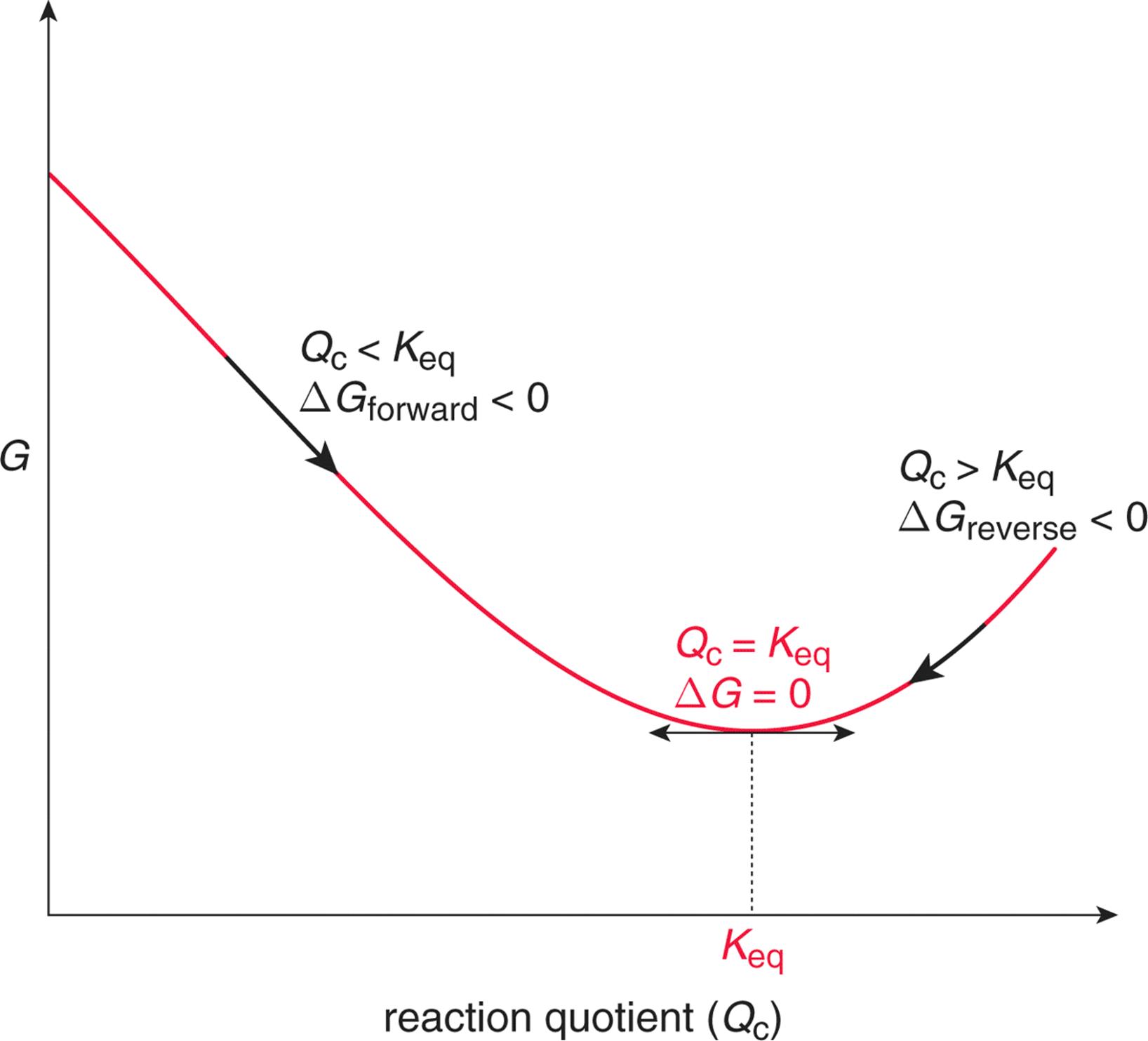
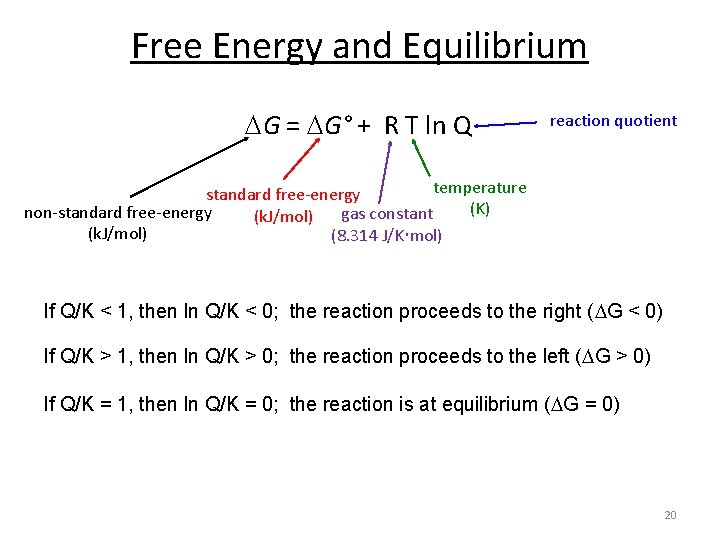
Where G is the difference in the energy between reactants and products. For example under standard conditions the reaction of Cos with Ni2 aq to form Nis and Co2 aq occurs spontaneously but if we reduce the concentration of Ni2 by a factor of 100 so that Ni2 is 001 M then the reverse reaction occurs spontaneously instead. Δ G Δ G ⁰ RT ln Q.
If the initial state is the standard state with P i 1 a t m then the change in free energy of a substance when going from the standard state to any other state with a pressure P can be written as follows. The Gibbs free energy equation is dependent on pressure. Free Energy and Free Energy Change the Gibbs free energy G is used to describe the spontaneity of a process.
ΔG ΔH TΔS. The equation is given as. The units of ΔG If you look up or calculate the value of the standard free energy of a reaction you will end up with units of kJ mol-1 but if you look at the units on the right-hand side of the equation they include J - NOT kJ.
G H - T D S. The second and fourth statements follow from combination of the first and second laws of. If ΔG 0 then K 1 and neither reactants nor products are favored at equilibrium.
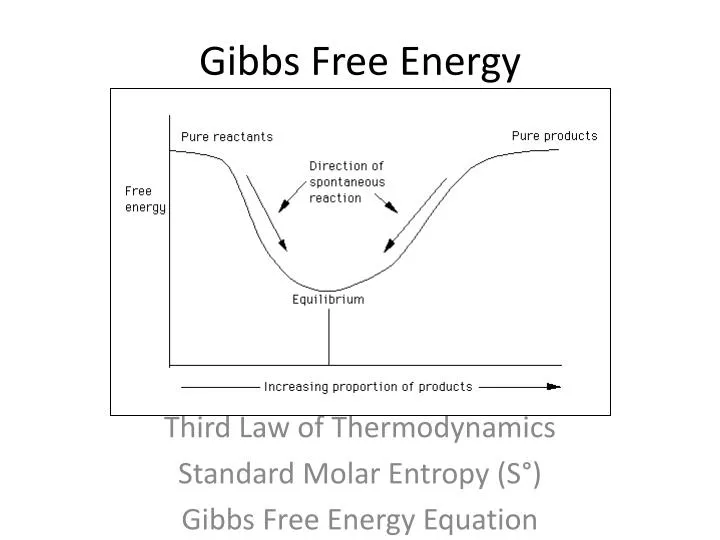
Gibbs Free Energy Equation A thermodynamic system is said to be in equilibrium if its intensive properties temperature pressure and extensive properties U G A are constant. G H - TS. UsingCell Potentials to Determine Non-standard State Free Energy Changes.
Ppt Gibbs Free Energy Powerpoint Presentation Free Download Id 2198185
Ib Chemistry On Gibbs Free Energy Equilibrium Constant And Cell Pote

Ap Chemistry Non Standard Gibbs Free Energy Worksheet Review Youtube

Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Practice Problems Youtube Free Energy Free Energy Projects Energy Technology

Chemical Thermodynamics Gibbs Free Energy Analytical Chemistry Video Clutch Prep

Standard Gibbs Energy An Overview Sciencedirect Topics

Reaction Quotient And Gibbs Free Energy At The Start Of A Reaction Chemistry Stack Exchange

1 The Laws Of Thermodynamics In Review 1 The Internal Energy Of An Isolated System Is Constant There Are Only Two Ways To Change Internal Energy Heat Ppt Download
Chapter 13 Principles Of Bioenergetics

Derivation Of Gibbs Free Energy Formula Chemistry Stack Exchange

Why Can We Write Pressure In Terms Of Gibbs Free Energy Anyone Knows How You Get To This Equation Chemistry
Chemistry The Central Science Chapter 19 Section 5

Gibbs Free Energy Example Video Khan Academy

Tang 05 Entropy And Gibb S Free Energy

Getting Gibbs Energy As A Function Of Temperature
How Is Gibbs Free Energy Related To Enthalpy And Entropy Socratic

Apsi 2018 Ap Chemistry 2 Chapter 3 2 By Edvantage Science Issuu
Gibbs Free Energy Equilibrium Thermodynamics

Standard Free Energy Changes Introduction To Chemistry

The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com
Solved Calculate The Change In Gibbs Free Energy At Stand Chegg Com

15 2 Gibbs Free Energy Hl Youtube
16 4 Gibbs Energy Chemistry Libretexts

Calculate The Standard Free Energy Of Reaction 1 Youtube

Free Energy And Pressure Concentration Ap Chemistry

The Standard Free Energy Change Of A Reaction Is Deltag Kj
Gibbs Free Energy Thermochemistry Training Mcat General Chemistry Review
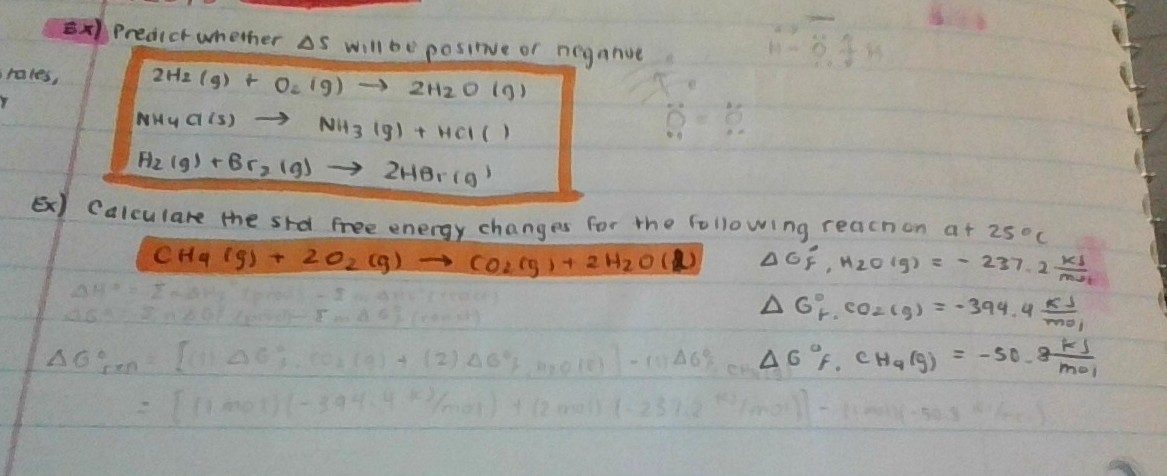
Calculate The Standard Free Energy Change For The Below Reaction At 25 Degrees Celsius Socratic
Going Green Free Energy And Equilibrium Equation
Solved Calculate The Change In Gibbs Free Energy At Stand Chegg Com

The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com

Ppt Free Energy And Redox Reactions Powerpoint Presentation Free Download Id 3198993

Relationship Between Free Energy And Equilibrium Constant Overview Video Chemistry Ck 12 Foundation

Free Energy Chemistry For Majors

Standard Gibbs Free Energy Ppt Download

Gibbs Free Energy Concept Chemistry Video By Brightstorm

Temperature In The Gibbs Free Energy Equation Chemistry Stack Exchange

Understanding Gibbs Free Energy Surfguppy Chemistry Made Easy For Visual Learners
Avoiding First Year University Chemistry Textbooks Misrepresentations In The Teaching Of Spontaneous Reactions

Determined To Succeed Teaching Chemistry Chemistry Education Chemistry Notes

Free Energy And Redox Reactions Ppt Download

Going Green Free Energy And Equilibrium Equation

Solution Calculate The Standard Free Ene Chemistry
Gibbs Free Energy Introductory Chemistry 1st Canadian Edition
Chapter 17 1 Gibbs Free Energy For A

Gibbs Free Energy Temperature And Spontaneity Ppt Download
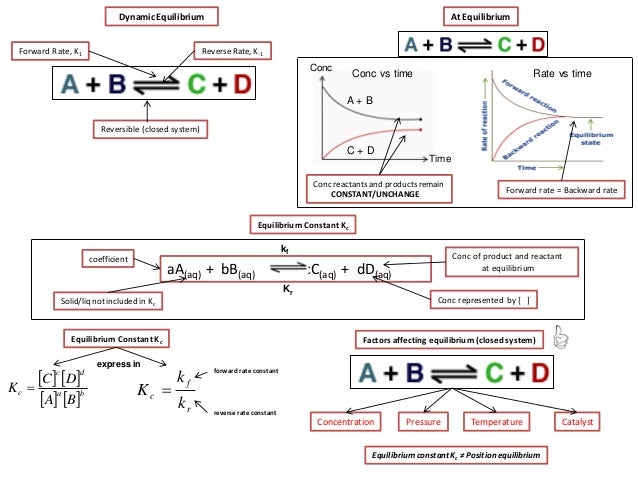
Ib Chemistry On Gibbs Free Energy And Equilibrium Constant Kc
First Some Cool Movies Here Is An Animated Form Of The Hcn Cnh Isomerization Click Here For The Isomerization Movie Here Is An Animated Form Of The Imaginary Frequency Of The Transition State Click Here For The Imaginary Frequency Movie Parameter
Gibbs Free Energy Thermochemistry Training Mcat General Chemistry Review

6 2 Potential Kinetic Free And Activation Energy Texas Gateway

Free Energy Delta G And Equilibrium Pt 8 Youtube

Atp Hydrolysis Gibbs Free Energy Video Khan Academy

17 1 Equilibrium And Gibbs Free Energy Hl Youtube

Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Free Energy Generator Free Energy Projects Free Energy

Ppt Free Energy And Redox Reactions Powerpoint Presentation Free Download Id 3198993

The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com
Chapter 13 Principles Of Bioenergetics
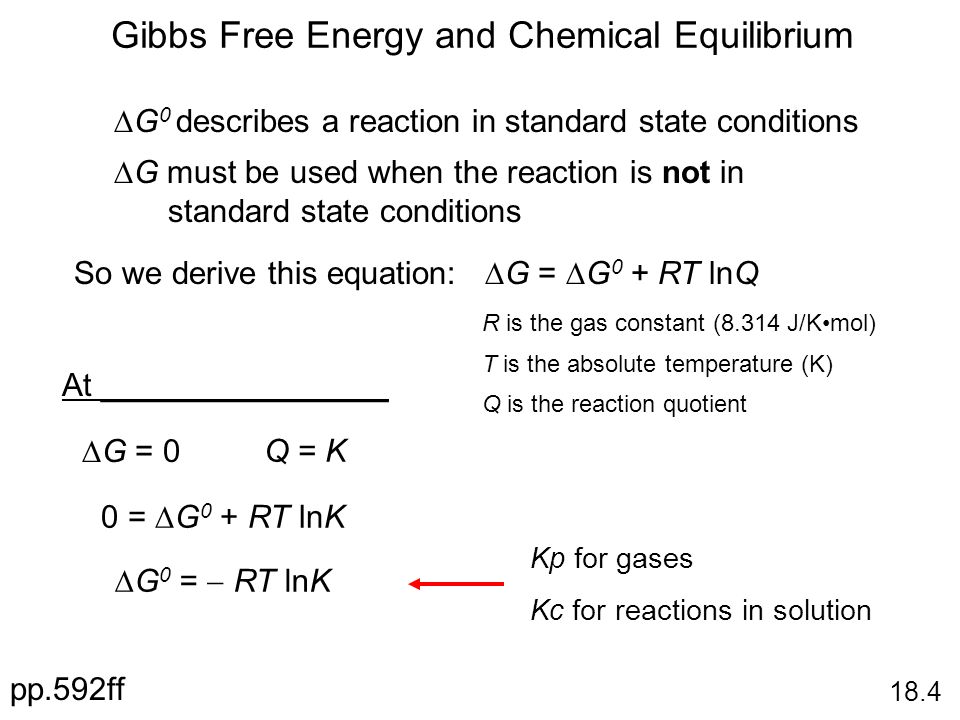
Thermodynamics Entropy Free Energy And Equilibrium Ppt Download
Chemistry The Central Science Chapter 19 Section 5

Chapter 19 7 Gibbs Free Energy Under Nonstandard Conditions Dg Vs K And Q And Coupled Reactions Youtube
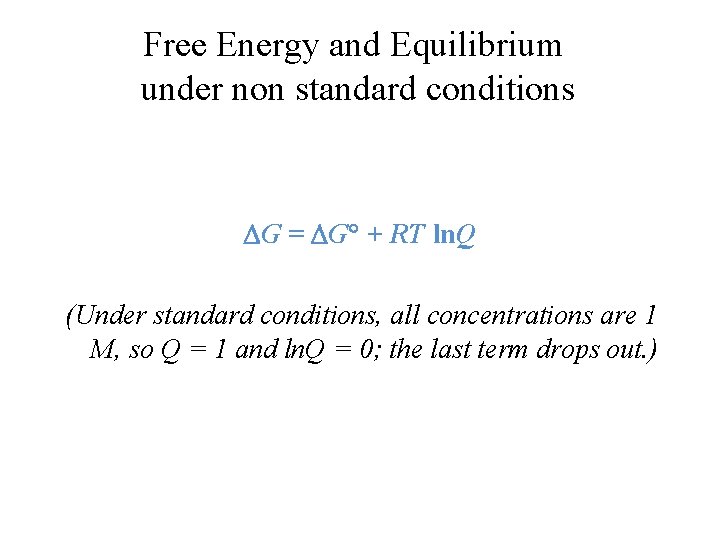
Gibbs Free Energy How Can We Use H

Gibbs Free Energy Dg Dh Tds Chad S Prep
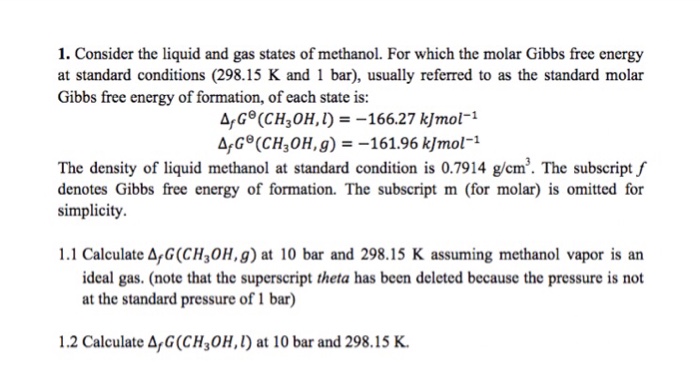
Solved 1 Consider The Liquid And Gas States Of Methanol Chegg Com
Free Energy And Cell Potential Video Khan Academy

Standard State Gibbs Free Energy Vs Nonstandard State Gibbs Free Energy Thermodynamics Chemistry Youtube
Solved 1 Consider The Liquid And Gas States Of Methanol Chegg Com

Difference Between Gibbs Free Energy And Standard Free Energy Compare The Difference Between Similar Terms

Calculate The Standard Change In Gibbs Free Energy Fo
Chemistry The Central Science 10 Th Edition Theodore
Chapter 17 1 Gibbs Free Energy For A

Free Energy Definition Biochemistry Going Green

Appendix C Standard Enthalpy And Gibbs Free Energy Of Reaction Fundamentals Of Chemical Engineering Thermodynamics Book
Solved Calculate The Change In Gibbs Free Energy At Stand Chegg Com

Gibbs Free Energy Boundless Chemistry

Gibbs Free Energy Of Formation An Overview Sciencedirect Topics
O Millesing 2 50 Pts In Chemistry 325 You Will Learn Or Learned That The Standard Gibbs Free Energy Change For A Chemical Reaction Is Course Hero
Chapter 17 1 Gibbs Free Energy For A

Temperature In The Gibbs Free Energy Equation Chemistry Stack Exchange

Tang 01b Enthalpy Entropy And Gibb S Free Energy Teaching Chemistry Chemistry Lessons Chemistry Classroom
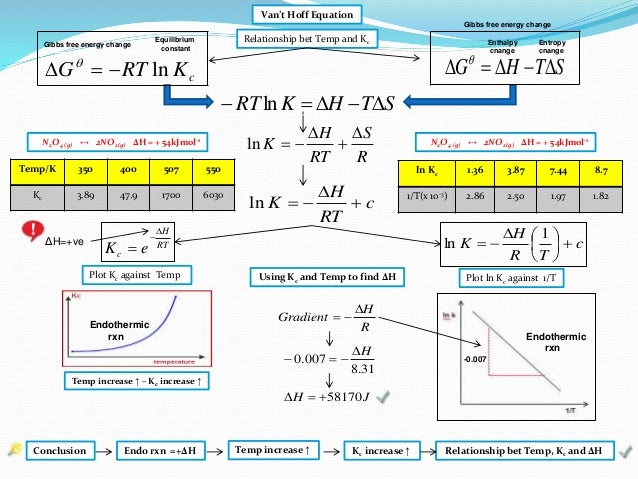
Going Green Free Energy And Equilibrium Equation
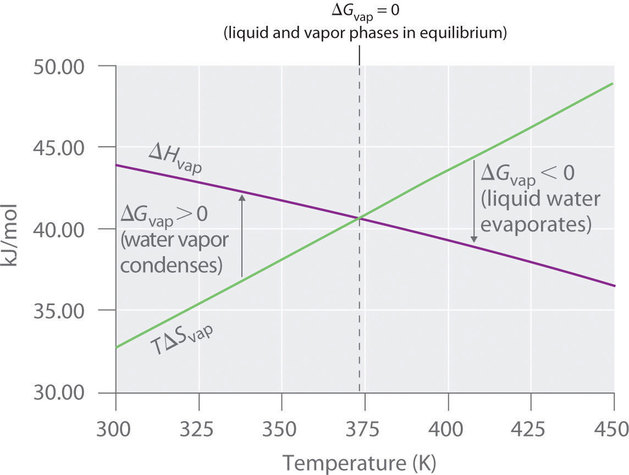
18 8 Gibbs Energy Changes In Chemical Reactions Chemistry Libretexts